Happy Earth Day everyone! This week, we will be focusing on some rock evidence that tells a story for how we humans impact our environment. It is a story of how we can negatively affect our environment and, most importantly, a demonstration of what we can achieve when we work towards a solution.
We are going to start in kind of a strange place this week: a graveyard! If you have ever walked through an old graveyard, you may have noticed that some gravestones appear to be so old and worn that it is difficult to make out any information about the person buried there. These heavily worn gravestones are most likely made of marble (the “in vogue” choice for gravestones during the 1800s)(1).

What is Marble?
Marble originally formed as a rock called limestone. Limestone is typically formed in ocean settings and is made up of calcium carbonate (the mineral calcite). Limestones form when the calcium carbonate-rich shells of marine organisms drop to the ocean floor or when calcite dissolved in ocean or even lake water precipitates out of the water (think of this as when you have a glass of salty or sugary water and the water evaporates, leaving behind a layer of salt or sugar at the bottom of your glass). Over time, this layer of calcium carbonate material is hardened into a rock called limestone. If that limestone gets squeezed under intense pressure and temperature, we get a metamorphic rock called marble.

Geologists love finding limestone or marble because we can pretty easily identify these rocks due to their reaction with acid! If you take a bit of acid (we generally use hydrochloric acid, or HCl, but be sure to follow all safety protocols when handling strong acids) and drop it on the limestone or marble surface, the calcium carbonate in the rock will react with the acid to form carbon dioxide bubbles. If you put enough acid on the rock surface, you will notice that bits of the rock will be dissolved. Evidence of this reaction (over many years) is what we are seeing when we notice marble gravestones that are heavily worn away. We can also notice the worn appearance of marble statues or even decorative marble on buildings. So, the big question is: what is the acid dissolving this marble? The answer? Acid rain.
Acid Rain
Rain is naturally more acidic than pure water (it has a pH value of around 5.6 while pure, distilled water has a neutral pH of around 7.0). However, “acid rain” has a pH value of 4.2 to 4.4 (remember, a lower pH value = more acidity). The lower pH of acid rain accelerates the dissolution of the marble gravestones and statues.
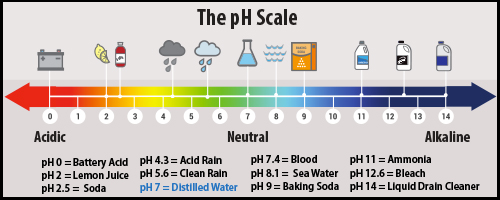
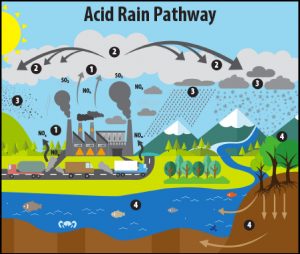
Acid rain is formed when pollutants such as sulphur dioxide (SO2) and nitrogen-bearing oxides (generally referred to as NOx) combine with water in the atmosphere to form sulfuric acids and nitric acids(2). Where do these pollutants come from? While some natural sources (like volcanoes) can periodically contribute large amounts of SO2 into the atmosphere, the main source for SO2 and NOx since the late 1800s has been human-generated emissions (pollutants from electricity generating power plants, vehicles, manufacturing, copper smelters, and other industries)(2)(3)(4).
Human Impacts on the Environment
Prior to the year 1970, there was little regulation of these emissions in the United States. However, the birth of the very first Earth Day in April 1970 helped lead to the formation of the Environmental Protection Agency (EPA) in December 1970(5). Over time, due to regulations put in place limiting the emission of SO2 and NOx, the amount of these pollutants in the atmosphere has decreased and we can see a trend (or pattern) of pH values for rainwater that has been less and less acidic(6)(7) (check out this site to look at data for the pH of rainwater in your area). This is great news! However, it took decades of hard work, research, and large systemic change to begin to reverse the trend of increasing rain acidity. Which leads us to our next question: what other environmental problems can we work together to find solutions for?

The acidification of rain due to human-related emissions leads us to consider one of the largest global crises affecting our Earth today: that of human-caused (or “anthropogenic”) climate change. We could write a blog post every day for the next 100 years on the importance and impacts of climate change (check out this list of climate change scientists, communicators, and activists as well as the climate change research of our friend and colleague Holly Olivarez here for more inspiration and information!). Modern climate change is a result of the human influence on Earth’s climate largely due to the increasing amount of greenhouse gases (namely carbon dioxide, methane, nitrous oxides, and chlorofluorocarbons) in our atmosphere resulting from the burning of fossil fuels, changes in land use, and other industrial factors(8). Climate change is global, but its societal impacts often more largely affect marginalized communities(9). We can see these impacts today in, for example, diminishing sea ice and coastal erosion in Native Alaskan villages(10), groundwater vulnerability in the Maldives(11), and increases in the number of climate refugees(12).
Examining the effects of acid rain on marble gravestones or statues in your own backyard might seem like a small-scale look at the impacts humans have on the environment. However, we can use what we know and our own experiences to learn about the global scale issues of climate change and make the individual and societal changes needed to address this issue pivotal to our world now and in the future. Start today on this 51st anniversary of the very first Earth Day!
1Appell, Jonathan. Stone Identification. Gravestone Preservation, 24 Feb. 2021, https://www.gravestonepreservation.info/articles. Accessed 21 April 2021.
2What is Acid Rain. Environmental Protection Agency, 12 May 2020, https://www.epa.gov/acidrain/what-acid-rain. Accessed 21 April 2021.
3Burns, Douglas A., et al. “Acid rain and its environmental effects: Recent scientific advances.” Atmospheric Environment 146 (2016): 1-4.
4Acid Rain and Water. United States Geological Survey, https://www.usgs.gov/special-topic/water-science-school/science/acid-rain-and-water?qt-science_center_objects=0#qt-science_center_objects. Accessed 21 April 2021.
5EPA History: Earth Day. United States Environmental Protection Agency, https://www.epa.gov/history/epa-history-earth-day. Accessed 21 April 2021.
6Driscoll, Charles T., et al. “Long-term temporal trends and spatial patterns in the acid-base chemistry of lakes in the Adirondack region of New York in response to decreases in acidic deposition.” Atmospheric Environment 146 (2016): 5-14.
7Acid Rain Program Results. United States Environmental Protection Agency, https://www.epa.gov/acidrain/acid-rain-program-results. Accessed 21 April 2021.
8The Causes of Climate Change. NASA Global Climate Change: Vital Signs of the Planet, 21 April 2021, https://climate.nasa.gov/causes/. Accessed 21 April 2021.
9Zhenmin, Liu, and Patricia Espinosa. “Tackling climate change to accelerate sustainable development.” Nature Climate Change 9.7 (2019): 494-496.
10Hamilton, Lawrence C., et al. “Climigration? Population and climate change in Arctic Alaska.” Population and environment 38.2 (2016): 115-133.
11Deng, Chenda, and Ryan T. Bailey. “Assessing groundwater availability of the Maldives under future climate conditions.” Hydrological Processes 31.19 (2017): 3334-3349.
12Climate change and disaster displacement. The United Nations High Commissioner for Refugees, https://www.unhcr.org/en-us/climate-change-and-disasters.html. Accessed 21 April 2021.

